Noninvasive optical inhibition with a red-shifted microbial rhodopsin
[Publisher Link] [Local Copy]
Chuong, A. S., Miri, M. L.*, Busskamp, V.*, Matthews, G.A.C.*, Acker, L.C.*, Soresnsen, A.T., Young, A., Klapoetke, N. C., Henninger, M.A., Kodandaramaiah, S.B., Ogawa, M., Ramanlal, S. B., Bandler, R. C., Allen, B. D., Forest, C.R., Chow, B.Y., Han, X., Lin, Y., Tye, K.M., Roska, B., Cardin, J.A., Boyden, E. S. (2014) Noninvasive optical inhibition with a red-shifted microbial rhodopsin, Nature Neuroscience 17:1123-1129. (*, equal contribution)
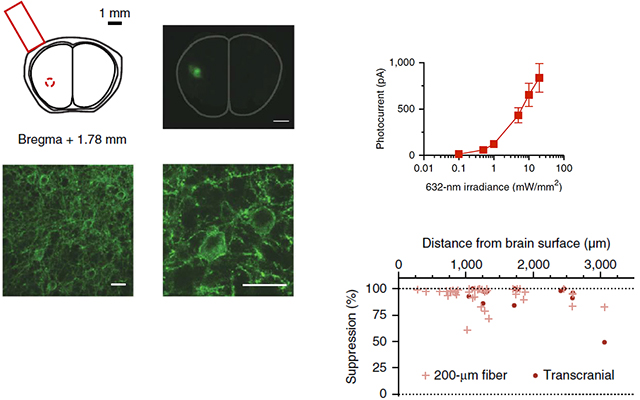
Optogenetic inhibition of the electrical activity of neurons enables the causal assessment of their contributions to brain functions. Red light penetrates deeper into tissue than other visible wavelengths. We present a red-shifted cruxhalorhodopsin, Jaws, derived from Haloarcula (Halobacterium) salinarum (strain Shark) and engineered to result in red light–induced photocurrents three times those of earlier silencers. Jaws exhibits robust inhibition of sensory-evoked neural activity in the cortex and results in strong light responses when used in retinas of retinitis pigmentosa model mice. We also demonstrate that Jaws can noninvasively mediate transcranial optical inhibition of neurons deep in the brains of awake mice. The noninvasive optogenetic inhibition opened up by Jaws enables a variety of important neuroscience experiments and offers a powerful general-use chloride pump for basic and applied neuroscience.
Resources associated with this Publication:
[Jaws: red light-drivable halorhodopsin]